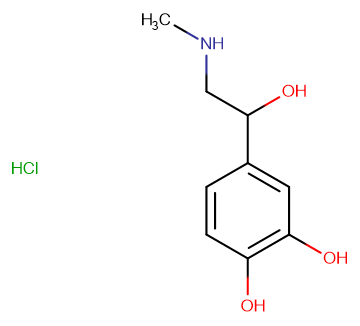
DL-Adrenaline Hydrochloride
CAS No. 329-63-5
DL-Adrenaline Hydrochloride( (±)-Adrenaline | DL-Adrenaline | DL-Epinephrine )
Catalog No. M14086 CAS No. 329-63-5
The active sympathomimetic hormone from the adrenal medulla in most species.
Purity : >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| Size | Price / USD | Stock | Quantity |
| 50MG | 34 | In Stock |


|
| 100MG | 45 | In Stock |


|
| 200MG | 52 | In Stock |


|
| 500MG | 84 | In Stock |


|
| 1G | Get Quote | In Stock |


|
Biological Information
-
Product NameDL-Adrenaline Hydrochloride
-
NoteResearch use only, not for human use.
-
Brief DescriptionThe active sympathomimetic hormone from the adrenal medulla in most species.
-
DescriptionThe active sympathomimetic hormone from the adrenal medulla in most species. It stimulates both the alpha- and beta- adrenergic systems, causes systemic vasoconstriction and gastrointestinal relaxation, stimulates the heart, and dilates bronchi and cerebral vessels.
-
In Vitro——
-
In Vivo——
-
Synonyms(±)-Adrenaline | DL-Adrenaline | DL-Epinephrine
-
PathwayAngiogenesis
-
TargetAdrenergic Receptor
-
RecptorAdrenergic Receptor
-
Research Area——
-
Indication——
Chemical Information
-
CAS Number329-63-5
-
Formula Weight219.67
-
Molecular FormulaC9H13NO3·HCl
-
Purity>98% (HPLC)
-
SolubilitySoluble in Water
-
SMILESCl.CNCC(O)C1=CC(O)=C(O)C=C1
-
Chemical Name——
Shipping & Storage Information
-
Storage(-20℃)
-
ShippingWith Ice Pack
-
Stability≥ 2 years
Reference
1. Leineweber K, et al. Naunyn Schmiedebergs Arch Pharmacol. 2007 Jul;375(5):303-9.
molnova catalog



related products
-
3,4,5-Trimethoxyphen...
Antiarol (3,4,5-Trimethoxyphenol) is a natural compound isolated from?Cochlospermum vitifolium.
-
Methoxyphenamine Hyd...
Methoxyphenamine is a β-adrenergic receptor agonist of the amphetamine class used as a bronchodilator.
-
Lofexidine
Lofexidine is a selective α2-receptor agonist Lofexidine reduces narcotic withdrawal symptoms.



 Cart
Cart
 sales@molnova.com
sales@molnova.com


